Most Potent Anti-Fatigue
Compound Ever Discovered
Re-printed from Allergy Research Group
Newsletter February 2004
See Additional Newsletter Articles on COBAT
Article Index
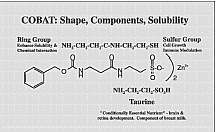
Taurox SB™ 6X Enhanced – formerly named “COBAT” – A Compound with
Novel Mechanisms of Action Emerges from Nanotechnology
(Taurox SB™ 6X Enhanced) “COBAT” is carbobenzoxy beta-alanyl-taurine,
a covalent chemical combination of taurine and
beta-alanine that has been modified to be efficiently absorbed via
the oral mucosa. It has a molecular weight of 724. Reports at scientific
meetings (Whole Person Healing Conference) document COBAT to be effective
in reversing fatigue (see graphs pages 4-6) in patients with chronic
fatigue, hepatitis C and cancer. The effective dose of COBAT is very
low, in the nanogram level making it, in our opinion, the most potent
and powerful general use anti-fatigue element ever discovered. In a
small clinical trial, COBAT was found to be effective
for reducing fatigue in approximately 90% of patients with chronic
fatigue, hepatitis C and cancer. The compound is so powerful and used
in such small amounts that it is considered a
"nano" compound from the concept of nano technology, and when compared to other
more commonly known anti-fatigue elements, it is consistently more than one
thousand to one million times more potent by weight. The mechanism of action is
definitely non-adrenergic; it modulates the immune system and is anticipated to
be complementary to large molecules (beta glucans, mush-room extracts,
etc.).
Cytokines Modulate Fatigue
It has been known that cytokines can strongly modulate
fatigue. For example, interferon-alpha (IFN-alpha), interleukin-2 (IL-2), or
tumor necrosis factor (TNF) have been used to treat cancer or hepatitis, and
they typically cause a "cytokine syndrome" of fatigue, fever, brain fog,
myalgia and depression. Such symptoms are similar to those that naturally occur
in these and other severe illnesses, hence these symptoms may be related to the
cytokines involved. An immune system response that is less than optimum may
result in cytokine imbalances – this is the fatigue or “cytokine
syndrome”.
IMMUNE MODULATION
COBAT is thought to potentate the immune response in T
cells as well as decrease tumor growth. Dunn, et al. examined the ability of
COBAT to modulate the immune system as detected by activation markers, second
messenger cascades, proliferation, and cytokine production, as well as in vitro
T cell cytotoxicity. The results of these studies gave insight into the
potential mechanism of action of COBAT as an immunostimulatory anticancer
agent. (See Table 1 below by Dunn, et al.)
TABLE 1: Summary of The Immunomodulation Effects of
COBAT (by Dunn, et al.)
| Direct Anti-Cancer
Effects |
Slowed Cell Growth of Human
Melanoma HT-144 Slowed Cell Growth of Human Melanoma SK-MEL-28 Did not
increase cell mortalities as do traditional chemotherapies |
| Components of T-Cell
Activation |
COBAT Performance |
Results |
| Cancer Cytoxicity |
Enhanced |
Stimulated Lymphocytes to kill
HL-60 promyelocytic tumor cells in vitro |
| Calcium Mobilization |
Enhanced |
Stimulates abrupt and substianed
(7 Minutes) calcium movements in PBMC's Similar results: B-Cell (Raji),
T-Cell (MOLT-4&H9), Monocytic cells (HL60) |
| CD 69 |
Increased |
Caused 150% increase in CD-69 for
T-Cells Caused 200% increase in CD-69 for T-Cells HUT-78, MOLT-4 |
| Surface TNF Alpha |
Increased |
Increased surface TNF Alpha 700%
to 130% (varied COBAT levels) Effected killing of HL 60 tumor cells in
vitro |
| Secreted TNF Alpha |
Decreased |
Reduced in stimulated
cells Supports COBAT limited toxicity and anti-allergy type effects |
| INF Gamma Message and
Protein |
Decreased Increased |
Normal - Increased IFN Gamma 480%
to 150% (varied COBAT levels) Normal - Decreased IFN Gamma 43% to 14%
(varied COBAT levels) |
| Proliforation with PHA |
Enhanced |
Stimulates T-Cell production 150%
- PHA present Appears to decrease or not affect cancer cell
proliferation |
| Proliforation without
PHA |
Enhanced |
Stimulates T-Cell production 200%
- PHA not present Appears to decrease or not affect cancer cell
proliferation |
| Killing Granules |
Enhanced |
Stimulates production of killing
granules. Enhances killing of cancer cells |
|
“In vivo anti-tumor mechanism of COBAT
may be through T-cell activation to increased cytokine production”
—Floyd Taub, M.D. |
 |
POTENT IMMUNE MODULATION
Tom Dunn at the University of Maryland, College Park,
performed numerous pharmacological experiments on COBAT, finding it to be a
potent immune stimulant for human cells. The increase in T-cell calcium flux,
which occurred at very low doses, and other immune function increases (see
Table 1, pg. 2) induced by COBAT suggested that stimulation of T cells might
contribute to its anti-tumor activity.
COBAT enhanced components of early T cell activation,
including increased up regulated expression of the CD69 T cell activation
marker and enhanced proliferation of blood peripheral mononuclear (immune)
cells in culture. T u m o r necrosis factor alpha (TNF-alpha) and interferon
gamma message RNA and protein were also up regulated in the presence of COBAT.
This up regulation was seen unless the cells were also exposed to excess levels
of exogenous stimulation, in which case a normalization of some abnormally high
values were seen. Thus COBAT is best referred to as an immunomodulator, as
opposed to an immunostimulator; it appears to produce a normalization or
“adaptogenic” response.
COBAT enhanced granzyme (esterase-containing killing
granules) in T cells, and also enhanced expression of cell surface TNFa. This
arming of lymphocytes with TNFa on the surface was accompanied by a decrease or
no change in the amount of secreted TNFa. Secreted TNFa may be associated with
systemic toxic effects. While surface TNF may provide significant benefits
without toxicity, Dunn et al. reported that the increased presence of surface
TNFa, mediated by COBAT , was able to effect the killing of HL60 tumor cells in
vitro. The in vivo anti-tumor mechanism of action of COBAT may be through T
cell activation in a calcium-dependent manner, leading to increased TNFa arming
on the surface of immune cells. The researchers also found similar arming of
monocytes.
The key factor that initiates the action of COBAT may
be the change in calcium flux it induces. This is a primary activation signal
for immune cells which lead to an amplifying cascade of immune stimulation
under the appropriate conditions. This phenomenon was observed at very low
(nanomolar) doses. Dunn suggested that COBAT may help keep the immune system
active in the elderly, as it is believed by some that diminished calcium
response is a factor in lowered T cell activity in older persons. The action of
COBAT is achieved at lower doses in vivo than in vitro. The ability of
nanomolar doses (one trillionth of a mole) of COBAT to alter calcium flux in
vitro is noteworthy.
ANIMAL MODELS
COBAT was shown to exhibit anticancer effects in
animals. It was shown to have potent anti-tumor effects against melanoma and
myeloma in animals at extremely low doses with negligible toxicity. The
compound was also tested in several animal models in which it stimulated immune
function and had extremely potent anticancer activity. (See Table 2 below)
TABLE 2: Animal Models of COBAT Activity
| Model (Test Site) |
Effectiveness |
| T-Cell and
B-Cell (Antibody) Stimulation |
| T-Cells Delayed Type
Hypersensitivity Mid Atlantic Bio Research |
Statistically significant
increase in both major and minor DTH response to ceasation. Responses observed
at doses as low as 1fg/kg utilizing in vitro T-Cell measurments. |
| Conjugate vaccine Viron
Systems |
Increase responses to
polysaccharide antigens; greater increases than the "Gold Standard" adjuvant
(CFA). Higher doses (5ug or 5mg/kg) were less effective than 5ng/kg performed
in mice measuring antiboby production. |
| Progressive
Cancer Models as Mono-therapy: Low Dose |
| Melanoma: Cloudman S-91
University New Mexico |
Increased survival; up to 80%
cure ; no survivors in control group. Doses ranged from fg/kg to ng/kg. Over
100 animals tested. G.D. Knight, et al. Cancer Research, 1994. |
| Melanoma: NS-1 University New
Mexico |
Increased survival with up to
100% tumor-free (100 pg/kg doses). 66%-75% survival at other doses (100 fg/kg
to 10 ng/kg) Small animal study. |
| Veterinary
Applications: (Cancer Mono-therapy): Low Dose |
| Fibrosarnoma: A
dog with fibrosarnoma was given 6 months to live. As of last update 20 months
from that timethe dog was not only alive, but, appears normal with no
reoccurance or side effects. |
| T-Cell Lymphoma:
Follow up on this dog included reports of greater quality of life, better than
expected liver enzymes, and less expected progression of disease. The small
tumors had resolved and no new tumors developed for many months and no reports
side efects. |
REDUCED FATIGUE
The Immunomodulator COBAT Reduces Fatigue in
Patients with Cancer, HCV and Chronic Fatigue Syndrome
Mayerson, S.E.
and Taub, F.E.
Late Breaking Presentation at the First
International Conference on Whole Person Healing, Bethesda, MD, March 28, 2003.
ACKNOWLEDGEMENT: The participating clinicians
(in alphabetical order) included: Toni Bark, M.D.; Franne Berez, M.D.; Zidi
Berger, M.D., N.D.; Burt Feldman, M.D.; Mitchell Fleisher, M.D.; Terri I.
Gilbert, N.D.; David Riley, M.D.; Tedde M. Rinker, M.D.; and David
Servan-Schreiber, M.D.
Reduced Fatigue Case
Studies
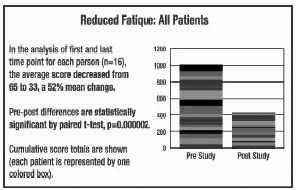
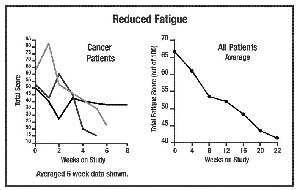 In the studies represented in
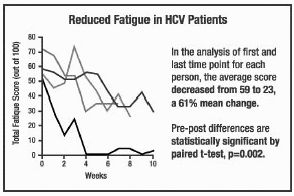
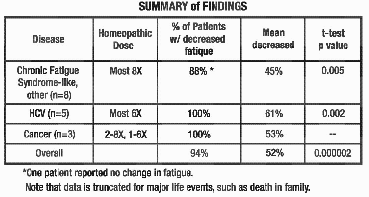
the graphs on pages 4-6, all but 1 out of 16 patients experienced a reduction
in fatigue. 52% was the average improvement. Significant test for pre-post
scores (p=.000002). See below for summaries from 5 of the 16 participants. In the studies represented in
the graphs on pages 4-6, all but 1 out of 16 patients experienced a reduction
in fatigue. 52% was the average improvement. Significant test for pre-post
scores (p=.000002). See below for summaries from 5 of the 16 participants.
 A 49-year-old male entered the
study with Chronic Fatigue Syndrome (CFS), fibromyalgia, allergies and oral
herpes. He took the COBAT dose for about 2 months. He reported increased
energy, no new eruptions of herpes and one cold that resolved more quickly than
usual. He noted some decrease in frequency of allergy and fibromyalgia
symptoms. A 49-year-old male entered the
study with Chronic Fatigue Syndrome (CFS), fibromyalgia, allergies and oral
herpes. He took the COBAT dose for about 2 months. He reported increased
energy, no new eruptions of herpes and one cold that resolved more quickly than
usual. He noted some decrease in frequency of allergy and fibromyalgia
symptoms.
A 39-year-old female with breast cancer and melanoma
entered the study for fatigue and PMS. She reported greater energy and better
sleep.
A 57 - year old female with arthritis and Irritable
Bowel Syndrome (IBS) reported less fatigue and improvement in IBS.
A 46 year old female with severe CFS and numerous
other conditions, including IBS, report-ed less fatigue and great improvement
in her IBS.
A middle-aged female with HCV, fatigue, and other
severe symptoms now wakes up without feeling "like a train wreck".
Reduced Fatigue Case
Studies (HCV)
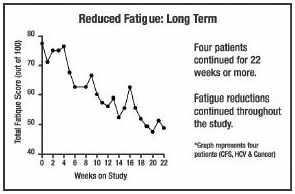 The following are summaries of
HCV patients who experienced decreased fatigue on the COBAT formula. The graphs
show changes in fatigue over time for various patients. The only intervention
given to these patients was COBAT. It was administered without nutritional or
other changes in patient activities. The following are summaries of
HCV patients who experienced decreased fatigue on the COBAT formula. The graphs
show changes in fatigue over time for various patients. The only intervention
given to these patients was COBAT. It was administered without nutritional or
other changes in patient activities.
A female patient experienced improvement with memory,
depression, headaches, and allergies. Another patient experienced less pain and
fewer GI symptoms.
 A middle-aged female with
Hepatitis C virus (genotype 1) entered the study due to fatigue. In 1999, she
had failed interferon alpha (IFN) therapy after an initial good response. IFN
caused severe debilitating side effects while providing only temporary stoppage
of viral proliferation. She took the COBAT formula for 4 months. Her viral load
decreased by about 40% on average, with a maximal decrease of 62%. She also
reported less fatigue on both doses. Her previously required daily naps became
unnecessary throughout the trial period. After she completed the trial and
stopped taking the COBAT formula, she frequently required lengthy daily naps. A middle-aged female with
Hepatitis C virus (genotype 1) entered the study due to fatigue. In 1999, she
had failed interferon alpha (IFN) therapy after an initial good response. IFN
caused severe debilitating side effects while providing only temporary stoppage
of viral proliferation. She took the COBAT formula for 4 months. Her viral load
decreased by about 40% on average, with a maximal decrease of 62%. She also
reported less fatigue on both doses. Her previously required daily naps became
unnecessary throughout the trial period. After she completed the trial and
stopped taking the COBAT formula, she frequently required lengthy daily naps.
Several patients with HCV also entered the trials with
elevated liver enzymes, all of which, decreased to normal during the trial.
These findings are considered interesting and support the hypothesis that
decreased fatigue is associated with changes in the immune system and decreased
liver damage. While the decrease in fatigue was documented, additional studies
are needed to document decreased damage to the liver.
Informal Trials
5 of 5
Respond!
In an informal trial done by one of the editors on
himself and 4 others (friends & co-workers) with mild fatigue, all
experienced complete resolution of fatigue in a very short period of time.
Below are what they had to say about COBAT:
Stephen Levine, Ph.D.:
“I found
personally that COBAT eliminated my long-standing early morning fatigue
presumably due to adrenal weakness.”
Diane Raile, CNC (ARG Clinical Nutritionist):
“Ten years ago I experienced the onset of Fibromyalgia, including
extreme overall body pain leading to chronic fatigue and depression. For three
years of that time I was almost completely non-functional and living on
disability. I have spent these past ten years with my main priority being,
regaining my health. I have done a variety of only non-allopathic methods and
treatments to identify and address the factors involved in my ill -ness, many
of which have been successful. Despite my success, I have felt that my "core",
for lack of a better description was still weak and fragile. Up until October,
2003 (when I began taking COBAT), I still found it necessary to sleep as many
as 12 to 14 hours a day, several days a month, or often an entire weekend, just
to be able to function. Five days after taking 15 drops of COBAT each morning
upon waking, I awoke feeling like a completely different person. I could feel
that my "core" was much stronger and that something had definitely shifted. I
can't explain it but this much deeper than just adrenals or mitochondria.”
Dixie Raile (Diane's twin sister):
“After a severe shoulder injury and a somewhat unsuccessful surgery
and rehabilitation, I have suffered from chronic pain. I start -ed into
college, and with the hours of studying and writing, the pain became
unbearable. I have never responded well to pharmaceutical medications so there
was nothing I could take. I wasn't sleeping well and between that and the
stress of the whole ordeal I was loosing ground fast. I was tired and depressed
and ready to give up. I tried COBAT and after 2 weeks I started waking up
feeling refreshed and had a better ability to concentrate. My attitude began to
improve which improved my experience of pain. I continue to take it everyday.
It seems like I have a solid foundation under me now; I'm more able to handle
stressful situations without falling apart and when I do fall apart, it doesn't
last as long.”
Claude Larson:
“COBAT is great.
It's very subtle, yet unmistakably effective. It creates no buzz or edginess,
yet it provides energy for both physical and mental work. It has allowed me to
stop drinking caffeinated coffee for the first time in my adult life."
Robbin Larson:
“Wow -when I began
taking COBAT, I started with only a few drops. I was amazed at the boost of
energy from such a low dose. It was very pleasant, not at all jittery as from
caffeine, and it lasted throughout the work day. Being menopausal, I found that
it decreased brain fog and increased my mental clarity. At the end of the day,
the quality of rest was also noticeable. I slept soundly and awoke refreshed
and relaxed“
Safety
Specific toxicology studies of COBAT suggest that it
has an unusually wide margin of safety between the effective dose and the
amount of material that can be harmful. Acute toxicology studies conducted by
Midlantic Bio-Research on COBAT in adult male and female CD-1 mice suggested a
maximally tolerated dose (MTD) of about 133 mg/kg administered as a single
intravenous bolus. A similar finding was obtained with rats. Thus, the toxic
dose levels of the compound appear high. The 6x strength of oral homeopathic
COBAT dosing is about one billion fold lower than this level. Studies of
oral COBAT (Product Safety Labs) found that rats tolerated 2000 mg/kg daily for
14 days without adverse effects. Water intoxication would prevent a person
from ingesting sufficient amounts of the preparation to receive even one ten
thousandth of the amount of COBAT anticipated to be toxic.
In a GLP (FDA-suitable good laboratory practices)
study in rats, no toxic side effects were observed following either oral or
subcutaneous 400 mcg/kg doses of COBAT given daily. During the 14-day
post-administration period, the animals all gained the appropriate amount of
weight as compared to saline-injected controls and there was no morbidity or
mortality. Following euthanasia, there were no abnormal findings in the gross
necropsies.
From human experience, we see that any material given
in sufficiently low doses, below the toxic animal dose, will also be non-toxic
in humans. Environmentalists and toxicologists consider that the low dose of
1/100,000 th of a compound's toxic dose is so safe that it can be labeled as
virtually non-toxic or virtually safe and does not need further analysis.
Questions about the COBAT formula? Contact our
Technical Support Department at: 800-545-9960
Focus on Allergy Research Group ®
Editor-in-Chief: Stephen A. Levine, Ph.D.
Managing Editor: Elise Zurlo, CNC
Medical Editor: Jeffry L. Anderson, M.D.
Assistant Copy Editors: Diane Raile, CNC &
Luba Voloshko, Ph.D.
FOCUS is published to provide scientific
information to healthcare practitioners and physicians. Certain persons
considered to be experts may disagree with one or more of the foregoing
statements concerning relationships of various nutritional factors to
structures and functions of the body, or various nutritional situations
adjunctive to certain bodily conditions. The same are deemed, nevertheless, to
be based on sound and reliable authorities. No such statements shall be
construed as claims or representations that any Allergy Research
Group®/NutriCology®, Inc. products, which are dietary supplements, are
offered for the diagnosis, cure, mitigation, treatment, or prevention of any
disease.
The statements made herein have not been evaluated by
the U.S. Food and Drug Administration. The products are not intended to
diagnose, treat, cure, or prevent any disease.
Copyright © 2003. Allergy Research Group®.
Special permission is required to reproduce by any manner, in whole or in part,
the materials herein contained. |



